There is a long debate that Supercapacitors will overrule the battery market in the future. A few years back when Supercapacitors were made available, there was a huge hype about it and many expected it to replace the batteries in commercial electronic products and even in Electric Vehicles. But, nothing like that actually happened, because both Supercapacitors and Batteries are entirely different from each other and they have their own applications. You can also check out the article on different types of batteries if you want to learn more about batteries in general.
Fun Fact: Almost all modern airbag controllers are powered by supercapacitors, because of their fast response time over batteries.
Compared with the battery, the Supercapacitor or Ultracapacitor is a high-density energy source or storage with huge capacitance for a short time span. In this article, we will discuss Supercapacitor vs Battery (Lithium / Lead Acid) on various parameters and conclude with a case study for an engineer to understand where one could select a supercapacitor over a battery for his applications. If you are a newbie to Supercapacitors then it is highly recommended to learn the basics of Supercapacitors before you proceed further.
Power Density
Supercapacitors have a high power density than the same rated battery. Although there are different kinds of batteries in the market, for example, lithium-ion, polymer, lead-acid batteries have different power density, from 1000 Wh per kg to 2000 Wh per kg. The ratings can also vary a lot depending on the manufacturing process. The comparison chart below shows the power density of Supercapacitor vs Battery.
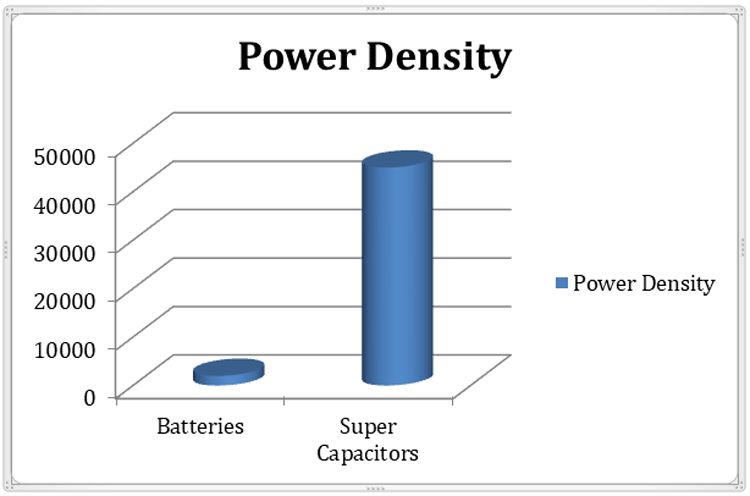
But, for a supercapacitor, the power density varies from 2500 Wh per kg to 45000 Wh per kg. That is much larger than the power density of the same rated batteries.
Due to the high power density, a supercapacitor is a useful power source where larger peak current is required.
Cell voltage
In different kinds of applications, often the input voltage is a large factor. Obviously, there are different kinds of voltage regulators available in the market but still, the input voltage across a regulator became an important part of the application. The below figure shows the output voltage of Supercapacitor vs Battery for the same number of cells.
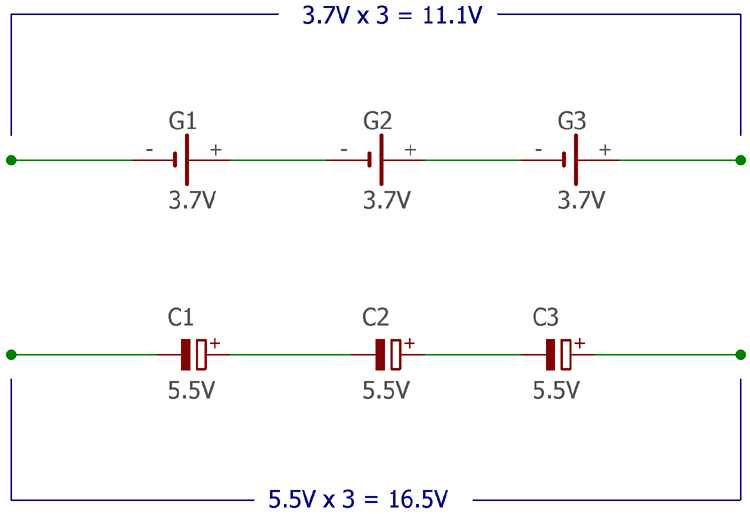
For example, an application with a linear voltage regulator like 7812 requires at least 15V input. A single-cell Lithium battery provides 3.2 volts at the lowest charge condition and 4.2 volts at the highest charge condition. Therefore, to compensate with the input voltage specification, at least 5 batteries in series connection is required but supercapacitor could provide 2.5 volts to 5.5-volt output. Supercapacitors have a high cell voltage of 5.5V compared with 3.7V of a typical Lithium battery. Thus, ignoring other limitations of a supercapacitor the circuit designer can choose three 5.5 volt supercapacitors in series. Over the battery, this is an undoubtedly plus point of supercapacitors in space constraint situations or cost optimization for purposes.
Efficiency
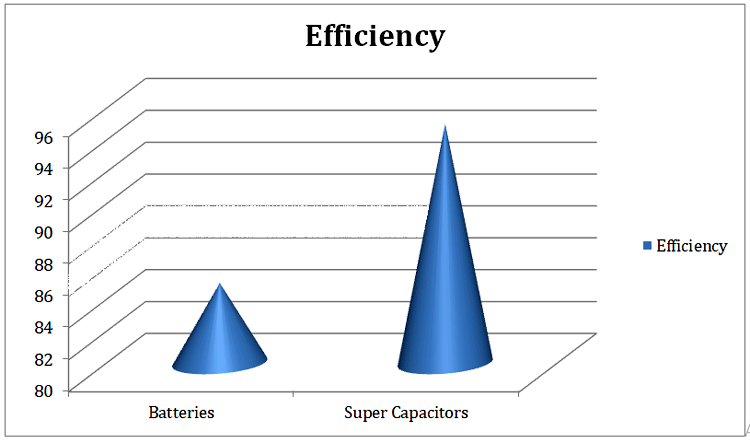
In terms of efficiency, supercapacitors are 95% more efficient than the batteries which are 60-80% efficient under full load conditions. Batteries in high load dissipate heat that contributes to low efficiency. Also, battery temperature and other parameters should be monitored during the charging and discharging using a Battery Management System (BMS), whereas in supercapacitors such strict monitoring systems might not be needed. The Efficiency of Ultracapacitor vs Battery is shown in the below figure. However, it should be noted that Supercapacitor also generates nominal heat during operation.
Reusability and Lifespan
Battery's lifespan is highly dependable on the charging and discharging cycles. In the case of Lithium and lead-acid batteries, the charging and discharging times are limited from 300 to 500 cycles, sometimes it can be a maximum of 1000 times. The lifespan without the charging and discharging situation lithium batteries can last for a span of 7 years.
A supercapacitor almost has infinite charge cycles, it can be charged and discharged for a huge number of times; it can be from 1 lakh to 1million of time. The lifespan of a supercapacitor is also high. A supercapacitor can last for 10-18 years, while a Lead-Acid battery can last around 3-5 years only.
Discharge Voltage Factor
A battery provides a relatively constant output voltage. But a supercapacitor output voltage decreases during discharging conditions. Therefore, while using batteries as a power source, one can use buck or boost regulator depending on the application requirements, but while using a supercapacitor, it is a popular choice to use a wide range boost converter for compensating the input voltage loss.
Charging Time
Different batteries use different charging algorithms. To charge Lithium-ion batteries constant voltage and constant current charging chargers are used. The charger needs to be specially configured to detect the charge condition of the battery as well as the temperature. For the case of lead-acid batteries trickle charging method is used.
Overall, to charge batteries irrespective of the Lithium-ion or lead-acid, it takes hours to get fully charge. The supercapacitor has supper fast charging time; it needs a very short period of time for getting a full charge. Therefore, for the applications where the charge time is required to be very less, supercapacitors definitely win over the same capacity of batteries.
Cost
Cost is an important parameter for product design related issues. Supercapacitors are a costly alternative when used instead of batteries. The cost sometimes gets very high such as 10 times higher when compared with the same capacity of the battery.
Risk factors
Lithium or lead-acid batteries require special care or attention during operating or charging conditions. Especially for lithium-ion batteries, the charging topology needs to be configured in such a way that the battery should not be overcharged or charged with a higher capacity of current than the battery can actually accept. This increases the risk of an explosion whenever the battery is overcharged or charged with a high current.
Not only in charging condition, but the batteries also need to be carefully operated during discharging situations. Deep discharge condition could potentially damage the battery life. Therefore, the battery needs to be disconnected from the load after it is reached to a certain level of charging state. Also, the short circuit of a battery is a dangerous situation.
Supercapacitors are safer than the batteries in terms of the above risk factors. However, charging a supercapacitor using a higher voltage than its rating is potentially harmful to the supercapacitors. But, when charging more than a single capacitor, it can become a complex job.
Case Study
Let's consider a situation where we want to light up 10 parallel LEDs for 1 hour. For this application, let's find out, as an engineer should we consider using a supercapacitor or lithium battery?
Let's assume the LEDs draw 30mA of current at 2.5V. Therefore, the wattage of 10 LEDs in parallel will be
2.5V x 0.03 x 10 = 0.75 Watt
Now, for 1 Hour of usage which is 3600 seconds, the energy required can be calculated as
3600 x 0.75 = 2700 Joules.
If we consider a 10F 2.5V Supercapacitor, it can store E = 1/2CV2 which is
½ x 10 x 2.52 = 31.25 Joules
Therefore, one needs at least 85 Supercapacitors in parallel with the same rating. Obviously in this specific application battery will be the first choice. But if this application changed into a specific application where the same amount of power is required only for 30 Seconds, Supercapacitor can be a choice as it can be charged very fast and can be used for a very long period of time.
Conclusion
The above comparison is only done between specific batteries (lithium or lead acid) with supercapacitors. However, there are different batteries with different chemical compositions. On the other hand, there are also different supercapacitors with different chemical compositions such as an aqueous electrolytic supercapacitor or with an ionic liquid supercapacitor as well as hybrid and organic electrolytic supercapacitors also in the market. Different compositions have different working characteristics and specifications.
Supercapacitors have much more positive points in terms of the application then the batteries. But, it also has negative sides compared with batteries. Therefore, the uses of supercapacitors are highly dependable on the type of application.





