Almost every portable and handheld device consists of a battery. The battery is a storage device where energy is stored to provide power whenever needed. There are different types of batteries available in this modern electronics world, among them Lead Acid battery is commonly used for high power supply. Usually Lead Acid batteries are bigger in size with hard and heavy construction; they can store a high amount of energy and are generally used in automobiles and inverters.
Even after getting competition from Li-ion batteries, lead-acid batteries are increasing day by day, because they are cheaper and easier to handle in comparison with Li-ion batteries. As per some market research Indian Lead Acid Battery Market is projected to grow at a CAGR of over 9% during 2018-24. So, it has huge market demand in Automation, Automotive, and Consumer Electronics. Although most Electric vehicles come with Lithium-ion batteries, there are still many electric two-wheeler which use Lead Acid batteries to power the vehicle. A deep exploration of lead acid battery working mechanisms is needed by engineers and technicians, and anyone associated with energy storage systems. This book covers in detail the lead acid battery construction and working, the chemical reactions involved, charging methods applied, and their actual applications.
In the previous tutorial, we learned about Lithium-ion batteries. Here, we will understand the Working, construction and applications of Lead Acid Batteries. We will also learn about charging/discharging ratings, requirements and safety of Lead Acid Batteries.
Table of Contents
Lead Acid Battery Construction
What is a Lead Acid Battery? If we break the name Lead Acid battery, we will get Lead, Acid, and Battery. Lead is a chemical element (symbol Pb the atomic number 82). It is a soft and malleable element. We know what Acid is; it can donate a proton or accept an electron pair when it is reacting. So, a battery, which consists of Lead and anhydrous plumbic acid (sometimes wrongly called lead peroxide), is called as Lead lead-acid battery. Understanding the construction of lead acid battery is crucial for grasping its working principle.
Lead Acid Battery Quick Reference
| Parameter | Specification | Details |
| Cell Voltage | 2.1V per cell | 12V battery = 6 cells |
| Charging Voltage | 13-14.4V (12V battery) | 10-15% above nominal voltage |
| Discharge Rating | 8-hour rate (C/8) | 160Ah = 20A for 8 hours |
| Operating Temperature | -20°C to 49°C | Optimal: 20-25°C |
Now, what is the internal construction?
A Lead Acid Battery consists of the following things, which we can see in the image below:
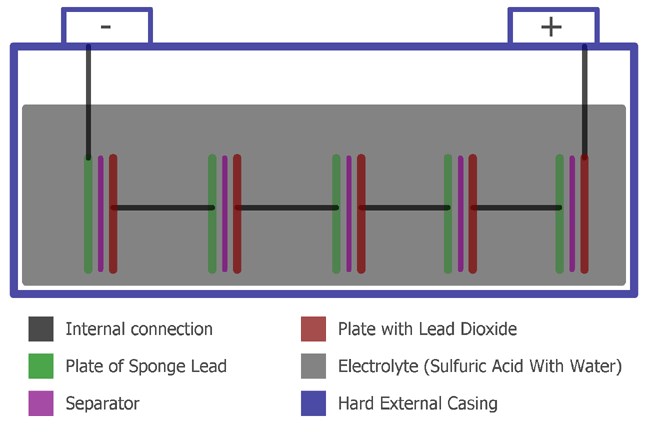
A Lead Acid Battery consists of Plates, a Separator, and Electrolyte, Hard Plastic with a hard rubber case.
In the batteries, the plates are of two types, positive and negative. The positive one consists of Lead dioxide, and the negative one consists of Sponge Lead. These two plates are separated using a separator, which is an insulating material. This total construction is kept in a hard plastic case with an electrolyte. The electrolyte is water and sulfuric acid.
The hard plastic case is one cell. A single cell store typically 2.1V. Due to this reason, A 12V lead acid battery consists of 6 cells and provides 6 x 2.1V/Cell = 12.6V typically.
Now, what is the charge storage capacity?
It is highly dependent on the active material (Electrolyte quantity) and the plate’s size. You may have seen that lithium battery storage capacity is described in mAh or milliamp-hour rating, but in the case of Lead Acid battery, it is amp-hour. We will describe this in a later section.
Working of Lead Acid Battery
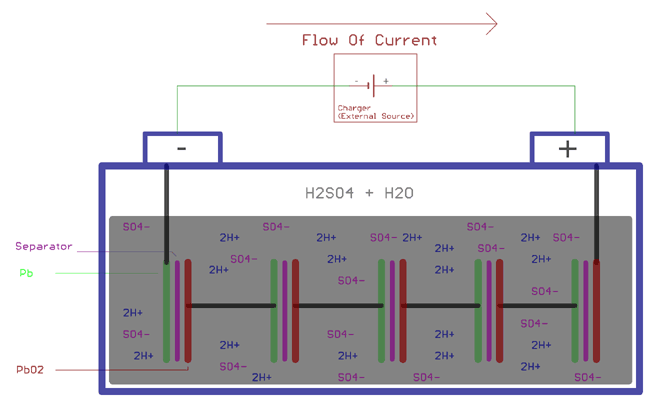
Working of the Lead Acid battery is all about chemistry, and it is very interesting to know about it. There are huge chemical process is involved in Lead Acid battery’s charging and discharging condition. The diluted sulfuric acid H2SO4 molecules break into two parts when the acid dissolves. It will create positive ions 2H+ and negative ions SO4-. As we told before, two electrodes are connected as plates, the Anode and the Cathode. The anode catches the negative ions, and the cathode attracts the positive ions. This bonding in the Anode and SO4- and the Cathode with 2H+ interchange electrons, and which further react with the H2O or with the water (Diluted sulfuric acid, Sulfuric Acid + Water). The lead acid battery working principle is based on reversible electrochemical reactions between lead compounds and sulfuric acid.
The battery has two states of chemical reaction: Charging and Discharging.
Lead Acid Battery Charging Process
As we know, to charge a battery, we need to provide a voltage greater than the terminal voltage. So to charge a 12.6V battery, 13V can be applied. Lead acid battery charging involves applying external voltage to reverse the discharge reactions.
But what actually happens when we charge a Lead Acid Battery?
Well, the same chemical reactions which we described before. Specifically, when the battery is connected with the charger, the sulfuric acid molecules break into two ions, positive ions 2H+ and negative ions SO4-. The hydrogen exchanges electrons with the cathode and becomes hydrogen. This hydrogen reacts with the PbSO4 in the cathode and forms Sulfuric Acid (H2SO4) and Lead (Pb). On the other hand, SO4- exchange electrons with the anode and become radical SO4. This SO4 reacts with PbSO4 of the anode and creates the lead peroxide PbO2 and sulfuric acid (H2SO4). The energy gets stored by increasing the concentration of sulfuric acid and increasing the cell potential voltage.
As explained above, the following chemical reactions take place at the Anode and the Cathode during the charging process.
At cathode
PbSO4 + 2e- => Pb + SO42-
At anode
PbSO4 + 2H2O => PbO2 + SO42- + 4H- + 2e-
Combining the above two equations, the overall chemical reaction will be
2PbSO4 + 2H2O => PbO2 + Pb + 2H2SO4
There are various methods applicable for charging the lead-acid battery. Each method can be used for a specific lead-acid battery for specific applications. Some application uses a constant voltage charging method, some application uses a constant current method, whereas trickle charging is also useful in some cases. Normally battery manufacturer provides the proper method of charging the specific lead-acid batteries. Constant current charging is not typically used in Lead Acid Battery charging.
The most common charging method used in lead acid batteries is the constant voltage charging method, which is an effective process in terms of charging time. In a full charge cycle, the charge voltage remains constant and the current gradually decreases with the increase of battery charge level.
Lead Acid Battery Discharging
Discharging of a lead acid battery is again involved with chemical reactions. The sulfuric acid is in the diluted form with a typical 3:1 ratio with water and sulfuric acid. When the loads are connected across the plates, the sulfuric acid again breaks into positive ions 2H+ and negative ions SO4. The hydrogen ions react with the PbO2 and make PbO and water, H2O. PbO starts reacting with the H2SO4 and creates PbSO4 and H2O.
On the other side, SO4- ions exchange electrons from Pb, creating radical SO4, which further creates PbSO4, reacting with the Pb.
As explained above, the following chemical reactions take place at the Anode and the Cathode during the discharging process. These reactions are exactly opposite of charging reactions:
At cathode
Pb + SO42- => PbSO4 + 2e-
At anode:
PbO2 + SO42- + 4H- + 2e- => PbSO4 + 2H2O
Combining the above two equations, the overall chemical reaction will be
PbO2 + Pb + 2H2SO4 => 2PbSO4 + 2H2O
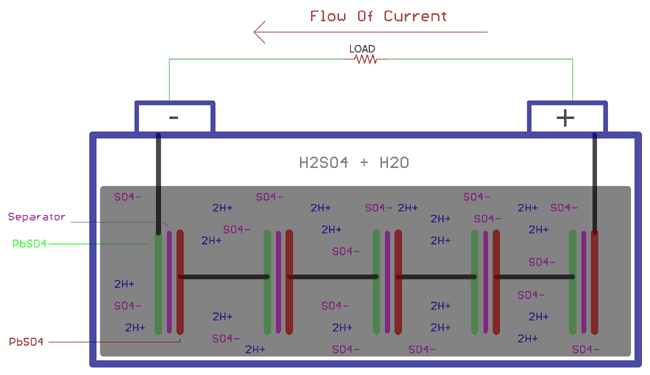
Due to the electron exchange across the anode and cathode, the electron balance across the plates is affected. The electrons then flow through the load, and the battery gets discharged.
During this discharge, the diluted sulfuric acid's gravity decreases. Also, at the same time, the potential difference of the cell decreases.
Risk Factor and Electrical Ratings
The lead acid battery is harmful if not maintained safely. As the battery generates Hydrogen gas during the chemical process, it is highly dangerous if not used in a ventilated area. Also, inaccurate charging severely damages the battery.
What are the standard ratings of a Lead Acid battery?
Every lead-acid battery is provided with a datasheet for standard charge current and discharge current. Typically, a 12V lead-acid battery, which is applicable for the automotive application, could range from 100Ah to 350Ah. This rating is defined as the discharge rating with an 8-hour timing period.
For example, a 160Ah battery could provide 20A of supply current to the load for 8 hours of the span. We can draw more current, but it is not advisable to do so. By drawing more current than the maximum discharge current in respect of 8 hours, the battery efficiency and the battery's internal resistance could also be changed, which further increases the battery temperature.
On the other hand, during the charging phase, we should be careful about the charger polarity; it should be properly connected with the battery polarity. Reverse polarity is dangerous for the lead-acid battery charging. The readymade charger comes with a charging voltage and a charging current meter with a control option. We should provide a greater voltage than the battery voltage to charge the battery. Maximum charge current should be the same as the maximum supply current at 8-hour discharging rates. If we take the same 12V 160Ah example, then the maximum supply current is 20A, so the maximum safe charging current is 20A.
We should not increase or provide a large charging current, as this will result in heat and increased gas generation.
Applications and Market Trends
| Application Sector | Typical Uses | Battery Requirements | Market Growth |
| Automotive | Starting, lighting, ignition (SLI) | High CCA, 12V, 40-100Ah | Stable demand |
| Industrial | UPS, backup power, forklifts | Deep cycle, 2V-48V systems | Growing 8-12% annually |
| Renewable Energy | Solar storage, wind power backup | Deep cycle, long life | Rapid expansion |
| Electric Vehicles | Two-wheelers, golf carts | Deep cycle, lightweight | Transitioning to Li-ion |
Lead Acid Battery Maintenance Rules
- Watering is the most neglected maintenance feature of flooded lead-acid batteries. As overcharging decreases water, we need to check it frequently. Less water creates oxidation in plates and decreases the lifespan of the battery. Add distilled or ionised water when needed.
- Check for the vents; they need to be perfected with rubber caps. Often, the rubber caps stick with the holes too tightly.
- Recharge lead-acid batteries after each use. A long period without recharging provides sulfating in the plates.
- Do not freeze the battery or charge it more than 49-degree centigrade. In cold ambient, batteries need to be fully charged, as fully charged batteries are safer than empty batteries with respect to freezing.
- Do not deep discharge the battery less than 1.7V per cell.
- To store a lead acid battery, it needs to be completely charged, then the electrolyte needs to be drained. Then the battery will become dry and can be stored for a long time.
Charging Methods for Lead Acid Batteries
| Charging Method | Description | Applications | Advantages |
| Constant Voltage | Fixed voltage, decreasing current | Automotive, UPS systems | Fast charging, prevents overcharge |
| Constant Current | Fixed current, increasing voltage | Laboratory, controlled environments | Precise control, uniform charging |
| Trickle Charging | Low continuous current | Standby applications | Maintains full charge, long-term storage |
| Smart Charging | Multi-stage automated process | Modern battery chargers | Optimized charging, extended battery life |
Frequently Asked Questions about the Construction of Lead Acid Battery
⇥ 1. How does a lead acid battery work?
A lead acid battery works by reversible actions of the two electrodes (lead dioxide (positive); sponge lead (negative)) and sulfuric acid as the electrolyte. In a normal discharge cycle, lead dioxide and sponge lead both convert into lead sulfate, and the battery produces current.
⇥ 2. What is the structure of a lead acid battery?
The structure consists of positive plates (lead dioxide) and negative plates (sponge lead), separators, sulfuric acid as the electrolyte, and a plastic case. Lead acid Batteries have six cells in series to give 12V.
⇥ 3. What are the cathode and anode reactions in lead acid batteries?
The anode (positive) at discharge is where PbO₂ is converted to PbSO,₄ and the cathode (negative) oxidizes Pb to PbSO₄. During charging the, processes are reversed to reform PbO₂ and sponge lead in the plates.
⇥ 4. What is the average lifespan of a lead acid battery?
The average lifespan of a lead-acid automotive battery is 3-5 years (most say not more than 5); a stationary battery (with some exceptions) can go from 5 to 15 years if well maintained. Life expectancy of a lead-acid battery is based on depth of discharge, state of maintenance, temperature, and charging method.
⇥ 5. What are the charging parameters for a lead acid battery?
When charging a 12V lead acid battery, a constant voltage charging of 13.8 - 14.4V is used. Anything above this voltage level will produce gassing and water loss, while less charging occurs below this voltage level.
⇥ 6. Can lead acid batteries be overcharged?
Yes. Overcharging a lead acid battery can create excessive gassing, water loss, heat and plate corrosion. An appropriate charge controller can be used to stop excessive charging, and charge with confidence once the voltage returns to below 14.4 volts in the case of 12V batteries.
Battery Applications in DIY Projects
Curious how batteries power our builds? Explore the project links provided below.
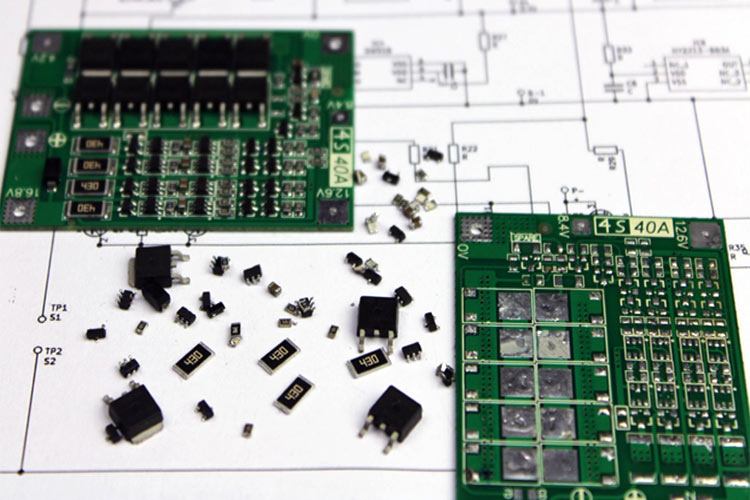
This comprehensive BMS circuit diagram guide explains the features and working of a 4S 40A Battery Management System (BMS) commonly used with 18650 Li-ion cells. We'll explore the complete BMS circuit for lithium-ion battery applications, including detailed schematics, component analysis, and protection mechanisms.
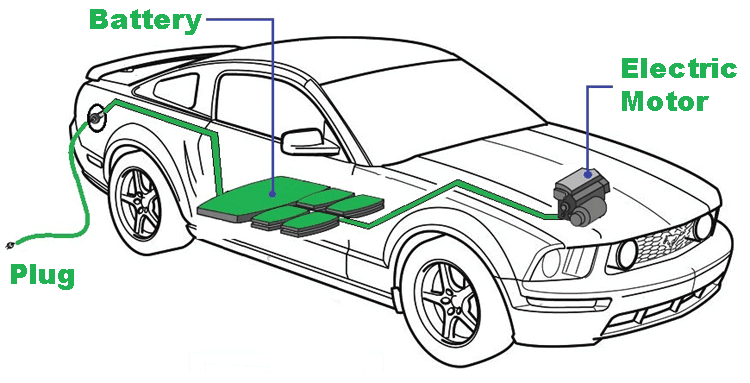
All you want to know about Electric Vehicle Batteries
In this article let us explore how an Electric Vehicle Battery Pack is designed for an EV and what are the vital parameters associated with batteries that has to be taken care of. You can also check out the article on different types of batteries if you want to learn more about batteries in general.
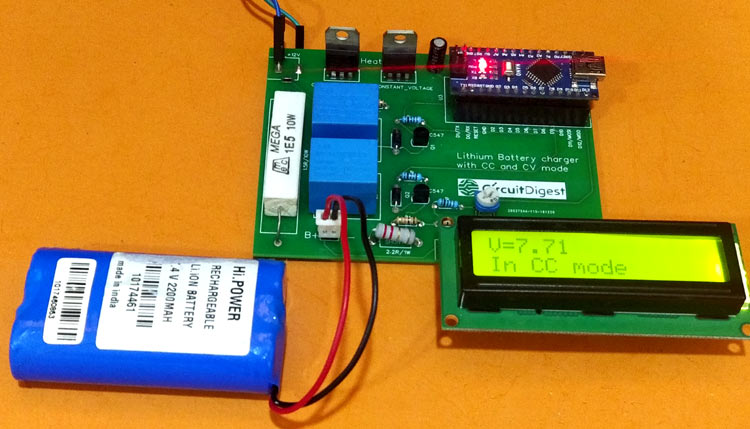
7.4V Two Step Lithium Battery Charger Circuit - CC and CV mode
In this project, we will build a two-stage battery charger (CC and CV) that could be used as to charge Lithium-ion or lithium polymer batteries. The battery charger circuit is designed for a 7.4V lithium battery pack (two 18650 in Series) which I commonly use in most robotics project but the circuit can be easily modified to fit in lower or slightly higher battery Packs like to build 3.7 lithium battery charger or 12v lithium ion battery Charger.