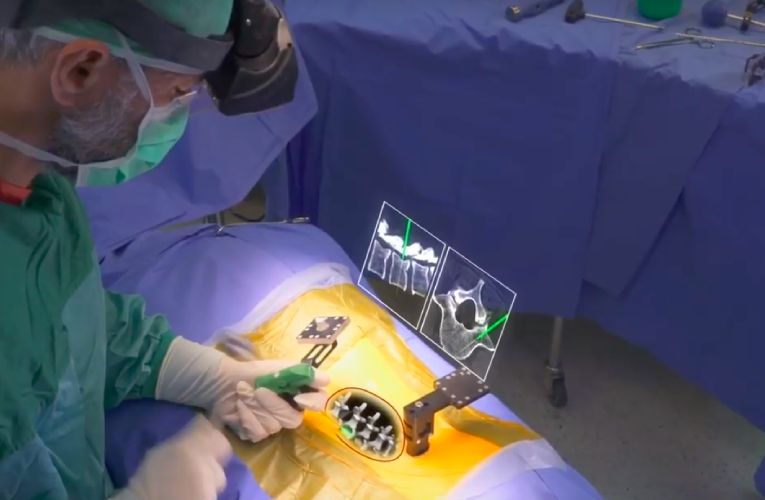
Augmedics, the creators of augmented reality (AR) surgical image guidance systems has announced the launch of its xvision Spine system (XVS). It has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its xvision augmented reality guidance system to be used in surgery. The groundbreaking advancement in the AR system will revolutionize surgery as it allows surgeons to visualize the 3D spinal anatomy of a patient in real-time as if it were X-ray vision. The vision allows surgeons to accurately navigate instruments and implants while looking directly at the patient instead of a remote screen.
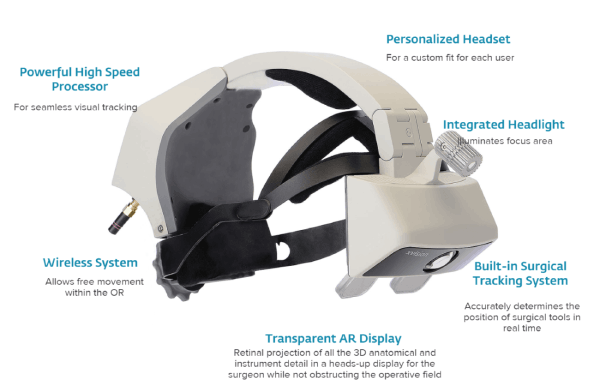
The xvision system is a wireless system that includes a transparent near-eye display headset and the elements of a traditional navigation system that helps in determining the position of surgical tools in real-time and superimposes a virtual trajectory on the patient’s CT data. The device projects 3D navigation data onto the surgeon’s retina, enabling him/her to simultaneously look at the patient and see navigation data without looking at a remote screen. Also, the integrated headlight illuminates the focus area.
A study of the xvision Spine system at Rush University Medical Center in Chicago positioned 93 screws in the thoracic and sacro-lumbar areas of five cadavers, comparing the actual screw tip position and trajectory to the virtual. Results revealed 98.9% accuracy using the Heary (thoracic) and Gertzbein (lumbar) scales. Augmedics launched the first-in-human clinical trial of its xvision Spine system in August 2018 and xvision is now available for sale in the US and Augmedics is expecting to start the distribution of headset in early 2020.

