These days Lithium-ion batteries are gaining more attention due to their widespread application in Electric Vehicles, Power backups, Mobiles, Laptops, smartwatches, and other portable electronic goods, etc. a lot of research is happening on lithium batteries with the increased demand for electric vehicles for much better performance. One important parameter that decreases the performance and lifetime of lithium battery is the development of a solid electrolyte interface (SEI), this is a solid layer that builds inside the lithium battery as we start using it. The formation of this solid layer blocks the passage between the electrolyte and electrodes heavily affecting the performance of the battery. In this article, we will learn more about this Solid electrolyte interface (SEI), its properties, how it forms and will also discuss how to control it to increase the performance and lifetime of a Lithium Battery. Note that some people also called Solid Electrolyte Interface as Solid Electrolyte Interphase (SEI), both the terms are used interchangeably overall research papers and hence it is hard to argue on which is the correct term. For the sake of this article, we will stick to the solid electrolyte interface.
Lithium-ion batteries:
Before we dive deep into SEI, let’s revise a bit on the basics of Li-ion cells so we better understand the concept. If you are completely new to electric vehicles then do check this All you want to know about Electric Vehicle Batteries article to understand EV batteries before you proceed further.
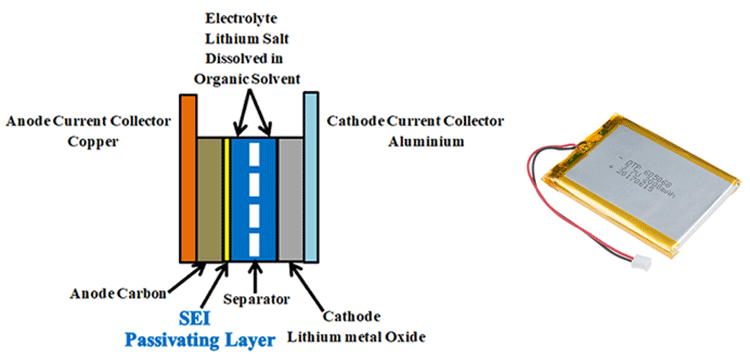
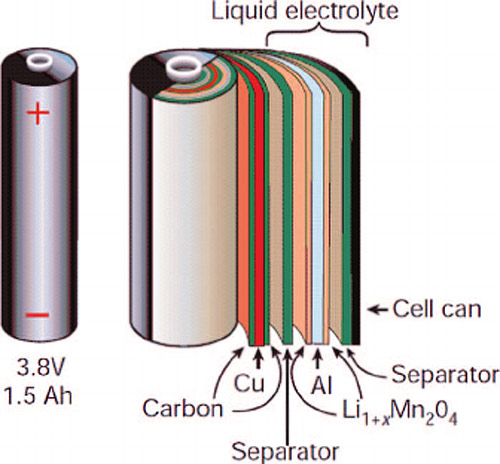
Lithium-ion batteries are made up of Anode (negative electrode), cathode (positive electrode), electrolyte, and separator.
Anode: Graphite, carbon black, lithium titanate (LTO), Silicon, and graphene are some of the most preferred anode materials. Most commonly graphite, coated on copper foil used as the anode. Graphite’s role is to act as a storage medium for lithium ions. Reversible intercalation of liberated lithium ions can be easily done in the graphite due to it’s loosely bonded layered structure.
Cathode: Pure Lithium having one valance electron on its outer shell is highly reactive and unstable, so that stable lithium metal oxide, coated on aluminium foil used as the cathode. Lithium metal oxides like Lithium nickel manganese cobalt oxide ("NMC", LiNixMnyCozO2), Lithium Nickel Cobalt Aluminium Oxide ("NCA", LiNiCoAlO2), Lithium Manganese Oxide ("LMO", LiMn2O4), Lithium Iron Phosphate ("LFP", LiFePO4), Lithium Cobalt Oxide (LiCoO2, "LCO") are used as cathodes.
Electrolyte: Electrolyte between the negative and positive electrodes must be a good ionic conductor and an electronic insulator that means it has to allow the lithium ions and has to block the electrons through it during the charging and discharging process. an electrolyte is a mixture of organic carbonate solvents such as ethylene carbonate or diethyl carbonate and Li-ion salts such as lithium hexafluorophosphate (LiPF6), lithium perchlorate (LiClO4), lithium Hexafluoroarsenate monohydrate (LiAsF6), lithium triflate (LiCF3SO3), and lithium tetrafluoroborate (LiBF4).
Separator: Separator is a critical component in the electrolyte. It acts as an insulating layer between anode and cathode to avoid the short circuit between them while allowing the lithium ions from the cathode to anode and vice versa during charging and discharging. In lithium-ion batteries mostly polyolefin is used as the separator.
Charging and Discharging Process
During the charging process when we connect a power source across the battery, energized Lithium atom, gives Lithium ions and electrons at the positive electrode. These Li-ions passes through the electrolyte and gets stored in the negative electrode, while electrons travel through the external circuit. During the discharge process when we connect external load across the battery, the unstable Li-ions stored in negative electrode travel back to the metal oxide at the positive electrode and electrons circulate through the load. Here aluminum and copper foils act as current collectors.
SEI formation:
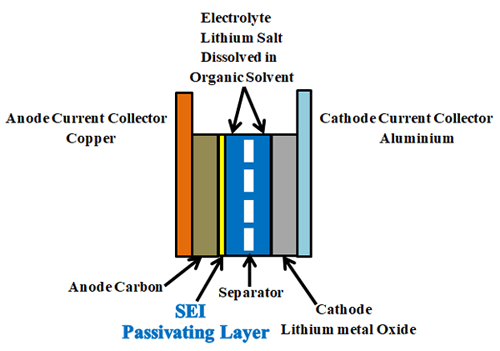
In Li-ion batteries, for the first charging, the quantity of lithium-ion given by the positive electrode is less than the number of lithium ions travelled back to the cathode after first discharging. This is due to the formation of SEI (solid electrolyte interface). For the first few charge and discharge cycles, when electrolyte comes in contact with the electrode, solvents in an electrolyte which are accompanied by the lithium ions during charging reacts with the electrode and starts to decompose. This decomposition results in the formation of LiF, Li2O, LiCl, Li2CO3 compounds. These components precipitate on the electrode and form a few nanometre thick layers called solid electrolyte interface (SEI). This passivating layer protects the electrode from the corrosion and further consumption of electrolyte, the formation of SEI occurs in two stages.
Stages of SEI Formation:
The first stage of SEI formation takes place before lithium ions inclusion into the anode. At this stage, unstable and highly resistive SEI layer forms. The second stage of SEI layer formation happens simultaneously with the intercalation of lithium ions on the anode. The resulting SEI film is porous, compact, heterogeneous, insulating to electrons tunneling and conductive for lithium ions. Once the SEI layer forms, it resists the electrolyte movement through the passivating layer to the electrode. So that it controls the further reaction between electrolyte and lithium ions, electrons at the electrode and thus restricts the further SEI growth.
Importance and Effects of SEI
SEI layer is the most important and less understood component in the electrolyte. Though the discovery of the SEI layer is accidental, but an effective SEI layer is important for the long life, good cycling ability, high performance, safety and stability of a battery. The formation of the SEI layer is one of the important considerations in the designing of batteries for better performance. Well adhered SEI on electrodes maintains good cycling ability by preventing further consumption of the electrolyte. The proper tuning of porosity and thickness of the SEI layer improves the lithium ions conductivity through it, results in improved battery operation.
During the irreversible formation of the SEI layer, a certain amount of electrolyte and lithium ions are permanently consumed. Thus the consumption of lithium ions during the formation of SEI results in a permanent loss of capacity. There will be SEI growth with the many repeated charges and discharge cycles, which causes the increment in battery impedance, temperature rise, and poor power density.
Functional Properties of SEI
SEI is unavoidable in a battery. however, the effect of SEI can be minimized if the layer formed adheres to the following
- It has to block the direct contact of electrons with electrolyte because contact between electrons from the electrodes and the electrolyte causes degradation and reduction of electrolyte.
- It has to be a good ionic conductor. It should allow the lithium ions from an electrolyte to flow to the electrodes
- It has to be chemically stable that means it cannot react with electrolyte and should be insoluble in the electrolyte
- It has to be mechanically stable which means it should have a high strength to tolerate the expansion and contraction stresses during charging and discharging cycles.
- It has to maintain the stability at various operating temperatures and potentials
- Its thickness should be close to a few nanometers
Controlling of SEI
Stabilization and control of the SEI are crucial for the improved performance and safe operation of the cell. ALD (atomic layer deposition) and MLD (Molecular layer deposition) coatings on electrodes control the SEI growth.
Al2O3 (ALD coating) with the bandgap of 9.9 eV coated on electrode controls and stabilizes the SEI growth due to its slow electron transfer rate. This will reduce the electrolyte decomposition and Li-ion consumption. In the same way Aluminium alkoxide, one of the MLD coatings controls the SEI layer build-up. These ALD and MLD coatings reduce the capacity loss, improves the coulombic efficiency.





